Explore the wave function in quantum theory: its role in particle behavior, impact on technology, and philosophical implications.
Understanding Wave Function in Quantum Theory
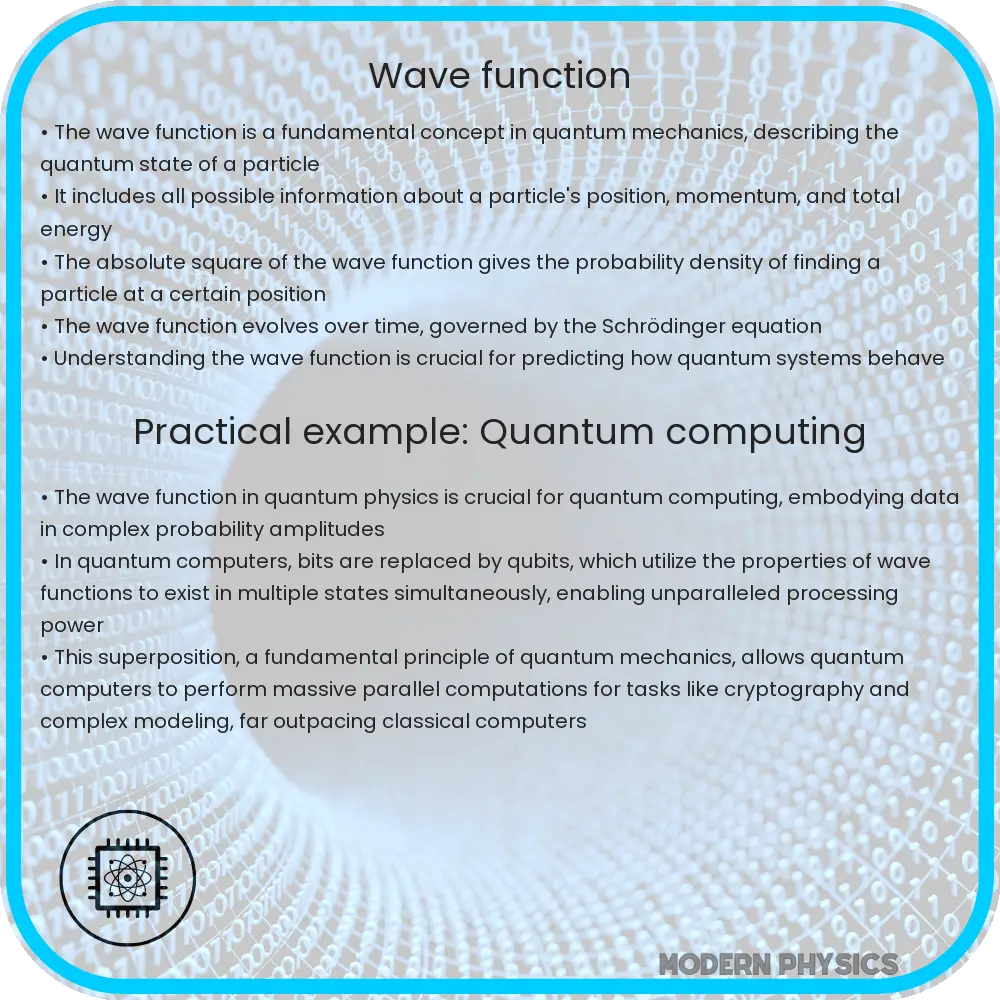
The wave function is a fundamental concept in quantum mechanics, representing the quantum state of a particle or system of particles. It is a mathematical function that provides the probabilities of the outcomes of a quantum system. In essence, the wave function is at the heart of quantum theory, playing a crucial role in describing the behavior of particles at the microscopic level.
Wave Function and Its Mathematical Representation
In mathematical terms, the wave function is denoted by the Greek letter Psi (Ψ). It is a complex function, meaning it can take complex values, and is defined for each point in space and time. The square of the absolute value of the wave function, |Ψ|^2, gives the probability density of finding the particle at a certain location in space at a given time. This probability interpretation was first introduced by Max Born, a key figure in the development of quantum mechanics.
Probability and Uncertainty in Quantum Mechanics
One of the most intriguing aspects of quantum mechanics is the inherent uncertainty in the behavior of particles. This is encapsulated in Heisenberg’s uncertainty principle, which states that certain pairs of physical properties, like position and momentum, cannot be simultaneously known to arbitrary precision. The wave function encapsulates this uncertainty, providing probabilities rather than definite predictions of a particle’s position and momentum.
Schrödinger Equation and Wave Function Evolution
The evolution over time of the wave function is governed by the Schrödinger equation. This fundamental equation in quantum mechanics describes how the quantum state of a physical system changes over time. In its simplest form, the time-dependent Schrödinger equation is written as:
iħ ∂Ψ/∂t = HΨ
Here, i is the imaginary unit, ħ (h-bar) is the reduced Planck constant, ∂Ψ/∂t is the partial derivative of Ψ with respect to time, and H is the Hamiltonian operator, representing the total energy of the system.
Wave Function Collapse
Another key aspect of quantum mechanics related to the wave function is the concept of wave function collapse. This occurs when a measurement is made on a quantum system, causing a transition from a superposition of states to a single eigenstate. This process is still a topic of much debate and research within the field of quantum physics.
The wave function, through its complex and abstract nature, challenges our classical intuitions about the physical world. It represents a core element of quantum mechanics, providing deep insights into the fundamental nature of reality at the smallest scales.
Applications of Wave Function in Quantum Theory
The applications of wave function in quantum theory are vast and diverse, impacting various fields such as chemistry, material science, and even information technology. In chemistry, for instance, the wave function is used to understand the electronic structure of atoms and molecules, which is essential for predicting chemical reactions and properties of materials. Similarly, in material science, it helps in studying the properties of semiconductors and superconductors, leading to advancements in electronic devices.
Wave Function in Quantum Computing
One of the most promising applications of wave function is in the field of quantum computing. Quantum computers use the principles of quantum mechanics, including superposition and entanglement, to perform calculations at speeds unattainable by classical computers. Here, the wave function describes the state of quantum bits (qubits), which are the basic units of information in quantum computing. The manipulation of these qubits, and hence the wave functions, is what allows quantum computers to solve complex problems more efficiently.
Philosophical Implications of the Wave Function
The concept of wave function also has profound philosophical implications, particularly in understanding the nature of reality and the debate over determinism versus probabilism in physics. The probabilistic nature of quantum mechanics, as conveyed by the wave function, suggests a fundamental randomness at the heart of reality, contrasting with the deterministic view of classical physics.
Challenges and Future Research
Despite its critical role in quantum theory, the wave function is not without its challenges. One of the biggest is the interpretation of quantum mechanics itself. Different interpretations, such as the Copenhagen interpretation, many-worlds interpretation, and pilot-wave theory, offer various explanations of the wave function and its role in the quantum world. Future research in quantum mechanics and quantum computing will continue to explore these interpretations and the fundamental nature of the wave function.
Conclusion
In conclusion, the wave function is a cornerstone of quantum mechanics, providing a mathematical framework for understanding and predicting the behavior of particles at the quantum level. Its implications stretch far beyond mere theoretical physics, influencing practical applications in multiple scientific and technological fields. The wave function’s inherent probabilistic nature challenges classical notions of reality and continues to fuel philosophical debates. As quantum technology advances, the wave function will undoubtedly play a pivotal role in shaping our understanding of the universe and driving future innovations.
