Understanding atomic transitions, the processes where atoms change energy states, crucial for advancements in spectroscopy, lasers, and quantum computing.
Introduction to Atomic Transitions
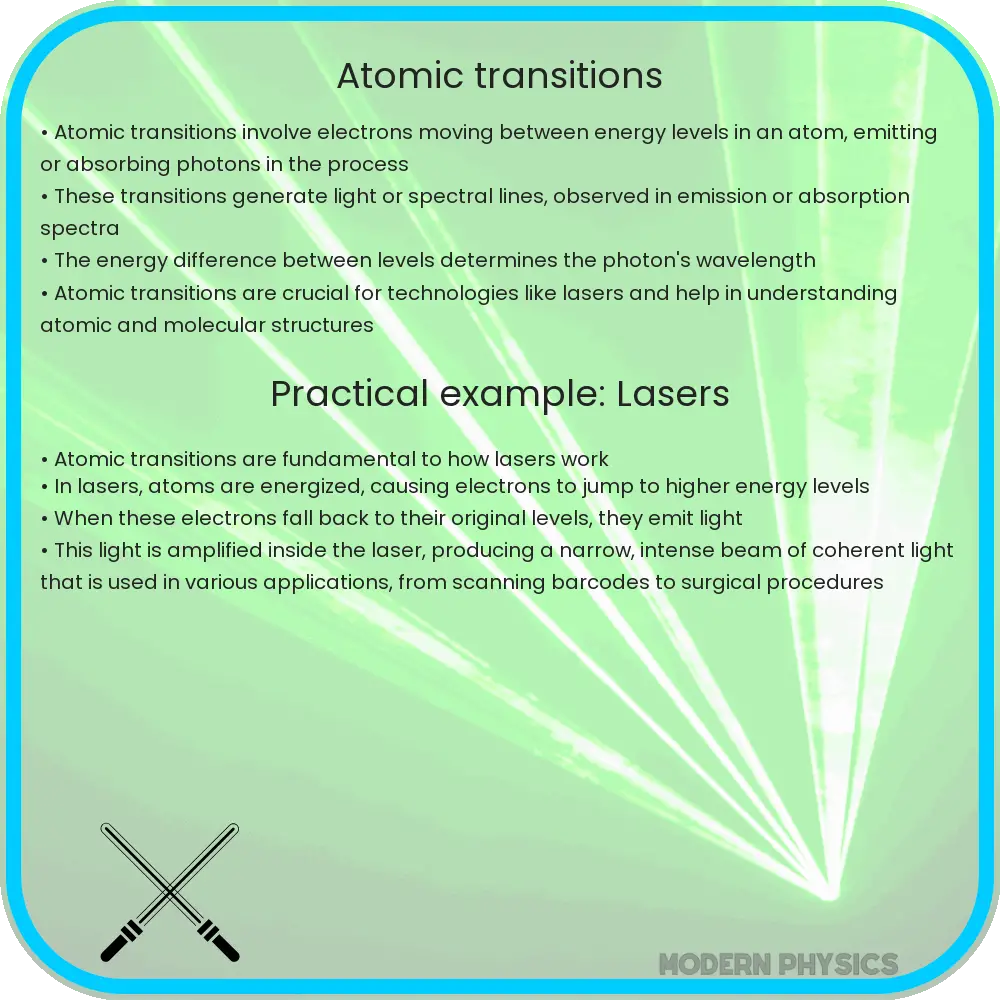
Atomic transitions are fundamental processes in which an atom changes from one energy state to another. These changes are crucial for the emission or absorption of electromagnetic radiation and play a pivotal role in various fields ranging from spectroscopy to the development of lasers and quantum computing. Understanding these transitions helps us to explore and manipulate the behavior of atoms and photons, fostering advancements in technology and scientific knowledge.
Types of Atomic Transitions
- Electron Transitions: The most common type involves electrons moving between energy levels within an atom. When an electron absorbs energy, it jumps to a higher energy level (excited state), and when it emits energy, it falls back to a lower energy level, releasing a photon with energy equal to the difference in energy levels.
- Vibrational Transitions: Primarily relevant in molecules, these involve changes in the vibrational state of a molecule. Energy changes due to vibration are typically observed in the infrared spectrum.
- Rotational Transitions: Also specific to molecules, rotational transitions involve changes in the rotational motion of a molecule and are generally observed in the microwave spectral region.
Mechanisms Behind Atomic Transitions
Atomic transitions occur through various interactions, with the most common mechanism being the absorption or emission of photons. This interaction is governed by quantum mechanical principles, particularly those outlined by quantum electrodynamics (QED). The probability of these transitions is dictated by selection rules, which determine the allowed transitions based on changes in quantum numbers associated with the atomic or molecular states.
The energy involved in an electron transition can be described by the equation E = h * nu, where E is the energy of the photon emitted or absorbed, h is Planck’s constant, and nu (ν) is the frequency of the photon. The difference in energy levels ΔE between the excited state and the ground state is reflected in the frequency or wavelength of the photon emitted or absorbed according to the relation:
E2 – E1 = ΔE = h * ν
Applications of Atomic Transitions
Atomic transitions have a wide range of applications:
- Spectroscopy: Used extensively in identifying the elemental composition of materials by analyzing the spectra produced by atoms at different energy states.
- Laser Technology: Fundamental in the operation of lasers, where a specific atomic transition is used to amplify light coherently.
- Quantum Computing: Quantum bits or qubits in quantum computers can be created through precise control of atomic transitions, enabling potentially revolutionary advances in computing power and security.
- Medical Imaging: Techniques like PET scans rely on the detection of photons from atomic transitions triggered by electron-positron annihilation.
Experimental Observation of Atomic Transitions
Atomic transitions can be observed and measured using several experimental techniques. One common method is absorption spectroscopy, where a sample is exposed to electromagnetic radiation, and the absorption at specific wavelengths indicates the occurrence of atomic transitions. Similarly, emission spectroscopy measures the light emitted by atoms or molecules, which allows researchers to determine the energy differences between quantum states.
Another technique, laser-induced fluorescence spectroscopy (LIF), involves exciting atoms or molecules with a laser and then detecting the photons emitted as they return to their lower energy states. This method is particularly sensitive and can be used to study dynamic processes in real time.
The Role of Atomic Transitions in the Environment
Atomic transitions not only have technological and scientific applications but also play a significant role in the natural world. For example, the ozone layer’s protection against harmful ultraviolet radiation relies on the absorption of this radiation, leading to molecular transitions in ozone that dissipate the energy harmlessly.
Similarly, atomic transitions explain many of the colors we see in nature. For instance, the vibrant colors of the aurora are caused by atomic transitions in the earth’s atmosphere, excited by solar wind particles.
Conclusion
Atomic transitions are not just abstract concepts but are integral to both the natural phenomena around us and the advanced technologies we utilize daily. From the deep blue of the sky, a result of light scattering and specific molecular transitions, to the precise operations inside a quantum computer, these transitions shape our understanding of the physical world.
Understanding these atomic and molecular changes opens the door to innovations in technology, such as more efficient energy sources and better diagnostic tools in medicine, making it a cornerstone topic in both physics and engineering. By studying atomic transitions, scientists and engineers continue to unlock new possibilities, paving the way for future advancements that may transform how we interact with the world around us.
